Epidemiology, Outcomes and Risk Management Scientists
Utilize longitudinal healthcare data that solves difficult problems in pre- and post-approval phases.
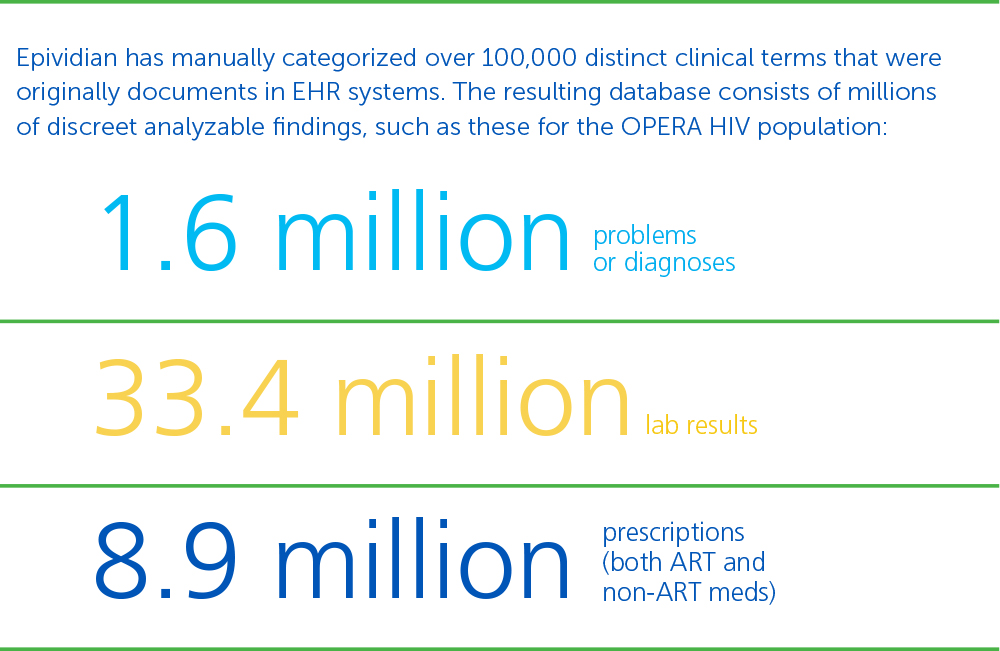
Learn how OPERA® (Observational Pharmaco-Epidemiology Research & Analysis) can improve clinical development and outcomes research. OPERA® is a longitudinal healthcare database containing anonymous patient-level data collected at the point of care. Using OPERA offers healthcare researchers the ability to apply sophisticated analytical techniques to longitudinal healthcare data.
From each practice’s CHORUS database, Epividian builds a large, de-identified longitudinal research database called OPERA. This OPERA database is used for research purposes (supporting both interventional and non-interventional studies) and to allow medical practices to anonymously benchmark against clinics from around the country to assess comparative metrics.
For the epidemiologist and outcomes researcher, this provides detailed clinical data for comparative effectiveness studies, comparative safety studies, post-approval commitments and other observational studies.
Risk-Benefit managers can use OPERA for information on the epidemiology of the indication as well as performing risk-benefit analyses required for regulatory reporting.
The clinical trialist can utilize OPERA and its direct connection to CHORUS to drastically reduce the time for feasibility and enrollment. OPERA provides a mechanism to address the most fundamental and difficult problems facing clinical trials in both pre- and post-approval settings.
The OPERA® solution offers services to support:
- Clinical Development: Phase I through IV
- Protocol feasibility
- Site yield evaluations
- Individual patient enrollment
Epidemiology, Outcomes Research & Risk Management
- Observational Cohort & nested Case-Control Studies
- Comparative Effectiveness Studies
- Health Outcomes
- Post Approval Safety Studies (PASS)
- Post Approval Effectiveness Studies (PAES)
Utilization & Trend Analysis
- Treatment patterns & outcomes
- Population-Treatment Dynamics
- Uptake & discontinuation trends
The Epividian technology platform and observational database program has been utilized by many physicians, epidemiologists and researchers to produce dozens of papers, abstracts and presentations.
Contact Epividian for information on how OPERA® can transform both your drug development and post-marketing support.